A new cancer immunotherapy strategy is described herein that combines the advantage of the well-established tumor targeting capabilities of high affinity recombinant fragments of antibodies (such as scFv) with the known efficient, specific, and potent killing ability of CD8 T lymphocytes directed against highly antigenic MHC/peptide complexes. Structurally, it consists of a new class of recombinant chimerical molecules created by the genetic fusion of scFv antibody fragments, specific for tumor cell surface antigens, to monomeric single-chain HLA-A2 complexes containing immunodominant tumor or viral-specific peptides.
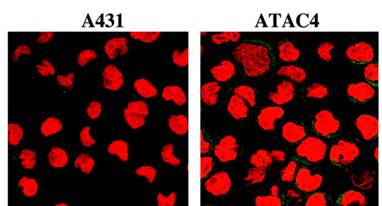
scHLA-A2/aTac(scFv) fusion folded around the G9-209M peptide to p55-positive ATAC4 but not to Tac-negative A431 cells. Lev A, Noy R, Oved K, Novak H, Segal D, Walden P, Zehn D, Reiter Y. Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):9051-6. Epub 2004 Jun 7.
When targeted to tumor cells expressing the relevant tumor-associated antigen, the fusion protein can induce very efficiently tumor cell lysis, regardless of the expression of self peptide-MHC complexes. Moreover, these molecules exhibited potent anti-tumor activity in vivo in nude mice bearing pre-established human tumor xenografts. When injected into tumor-bearing nude mice, followed by the transfer of tumor or viral-specific human CTLs, marked regressions of tumor xenografts were observed. These in vitro and in vivo results suggest that recombinant scFv-MHC-peptide fusion molecules could represent a new approach to immunotherapy, bridging antibody and T lymphocyte attack on cancer cells.